Dendritic Cell Therapy
Dendritic Cell Therapy
Dendritic cell therapy (DCT) is a highly personalised form of immunotherapy that harnesses the power of the body’s own immune system to fight cancer. Dendritic cells, often called the "generals" of the immune system, play a vital role in educating and directing immune cells, such as T-cells and natural killer cells, to recognise and attack cancer cells.
What are Dendritic Cells?
Dendritic cells are immune cells responsible for detecting and processing antigens, teaching the immune system what to target. In DCT, these cells are harvested from the patient’s blood, sensitised in the lab to recognise tumour-specific antigens, and then reintroduced into the patient’s body to initiate a potent immune response against cancer.
How are Dendritic Cells Obtained?
At Sanctura, we begin by extracting 200ml of blood from the patient. This blood is sent to a specialised lab, where dendritic cell progenitors are isolated. These progenitor cells are crucial in producing large numbers of dendritic cells. We use an advanced process to enhance the yield of these cells, which includes priming the patient's immune system with growth factors, mushroom extracts, and photobiomodulation techniques to optimise dendritic cell production.
The Manufacturing Process
Once the dendritic cells are isolated, they undergo a series of laboratory processes to ensure maximum potency:
Tumour Sensitisation
Proteins from the patient’s tumour (extracted from a biopsy) are broken down into peptides representing tumour-specific antigens. These peptides are then loaded onto the dendritic cells, priming them to recognise and attack the tumour.
Exosome Enrichment
Exosomes are extracellular vesicles that carry tumour antigens, further boosting the immune response. This added layer of immunotherapy improves the activity and efficacy of the vaccine by packaging these antigen signals more effectively.
Activation and Concentration
Before the dendritic cells are returned to the patient, they are activated using photobiomodulation at 680nm, which primes their mitochondrial activity. This step ensures the cells are fully functional upon injection and ready to stimulate a robust immune response.
How are Dendritic Cells Administered?
Once prepared, the dendritic cell vaccine is delivered subcutaneously or into lymph nodes. We administer the vaccine in four cycles:
Cycle One
Initial vaccine administration (along with photobiomodulation and hyperthermia).
Cycle Two
Second dose after two to three weeks.
Cycle Three
Third dose four weeks after the second.
Cycle Four
Final dose six weeks after the third.
Sanctura’s Approach to Dendritic Cell Therapy
Immune System Priming
We prime the immune system before the harvest using growth factors, mushroom extracts, and photobiomodulation to ensure higher yields of dendritic cells.
Exosome Enrichment
Our process includes exosome enrichment, packaging tumour antigens more effectively to amplify the immune response.
Mitochondrial Activation
Post-injection, we enhance dendritic cell function with 680nm red light therapy, activating the cells to their full potential.
Increased Dendritic Cell Yield
We ensure a higher concentration of cells (over 20 million per vaccine) than standard treatments, making the therapy more potent.
Treatment Schedule
Day One
Blood and tumour sample extraction.
Week Two
Vaccine production and first administration.
Months One - Four
Subsequent vaccine administrations monthly.
Month Six
Review and consider additional cycles.
Clinical Trials and Research
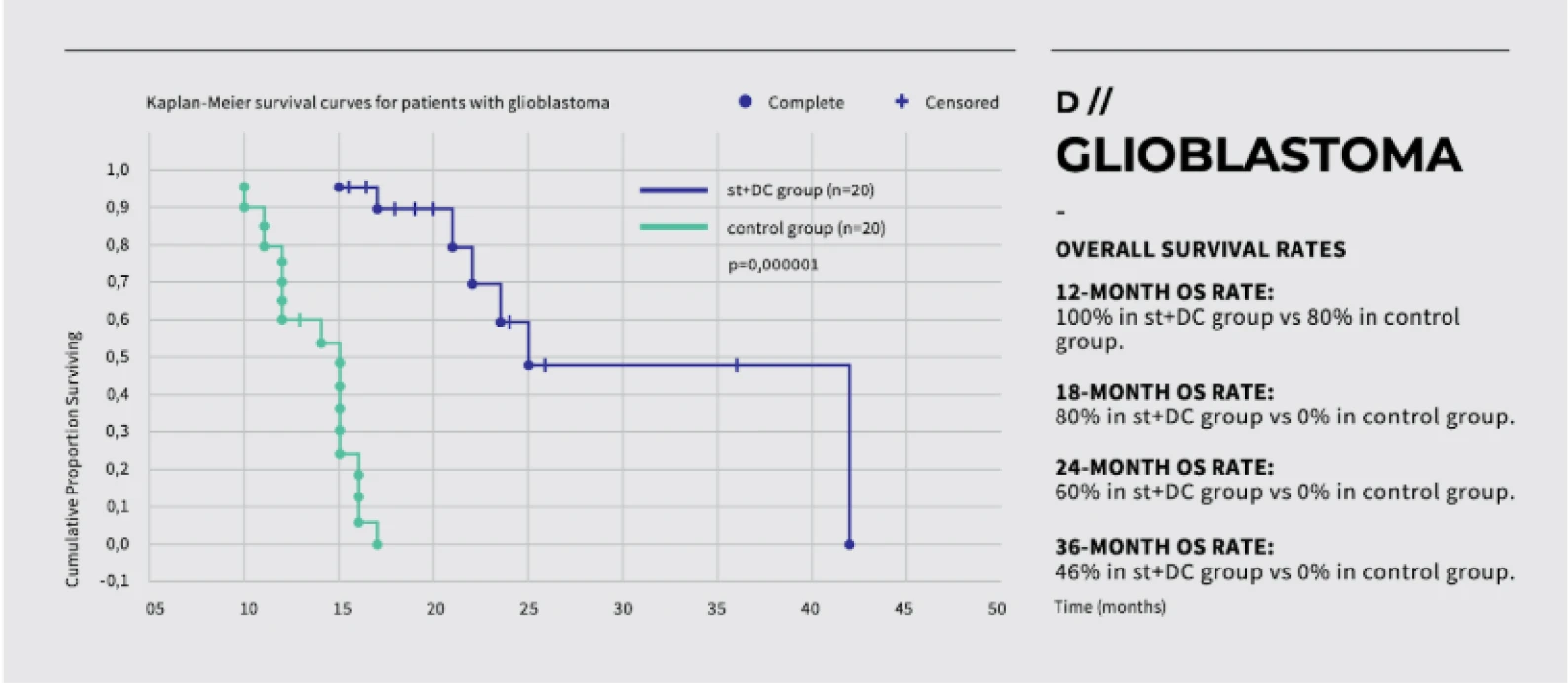
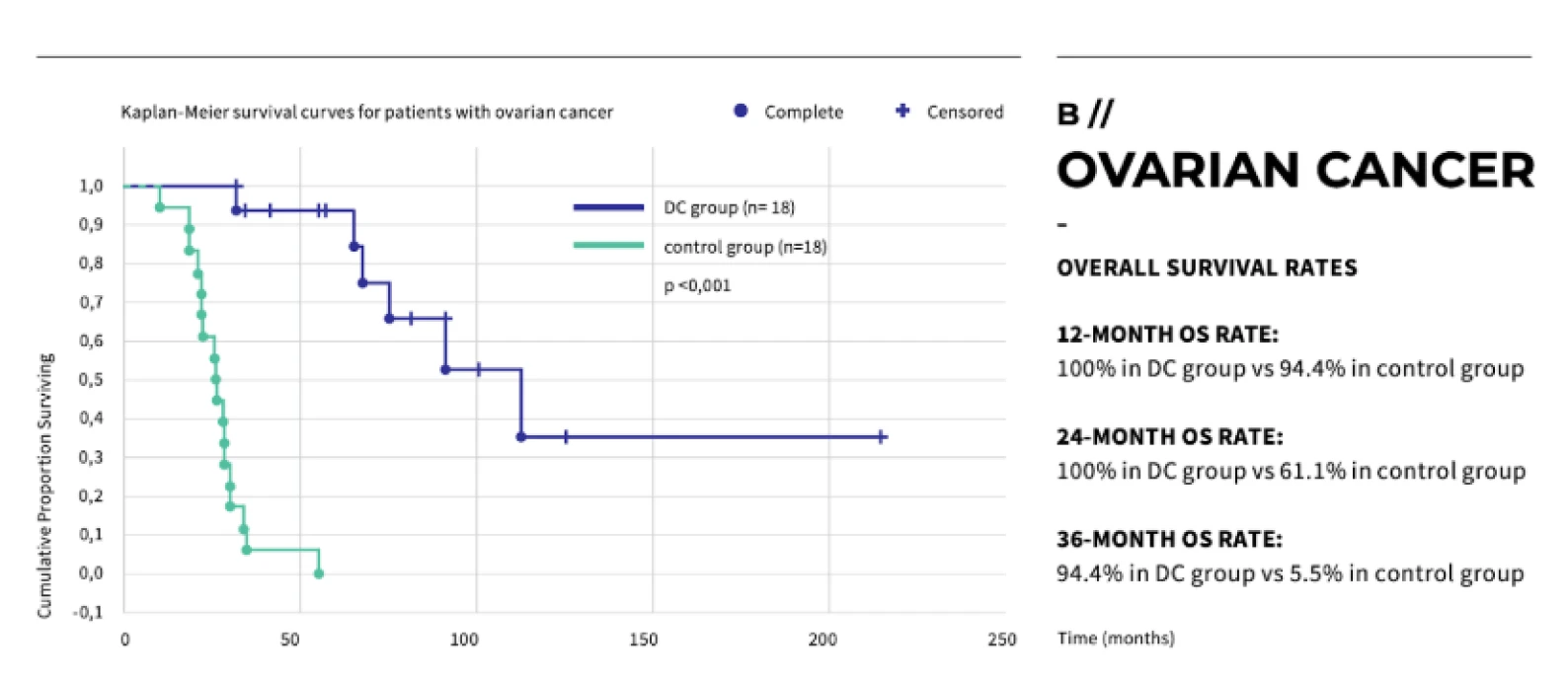
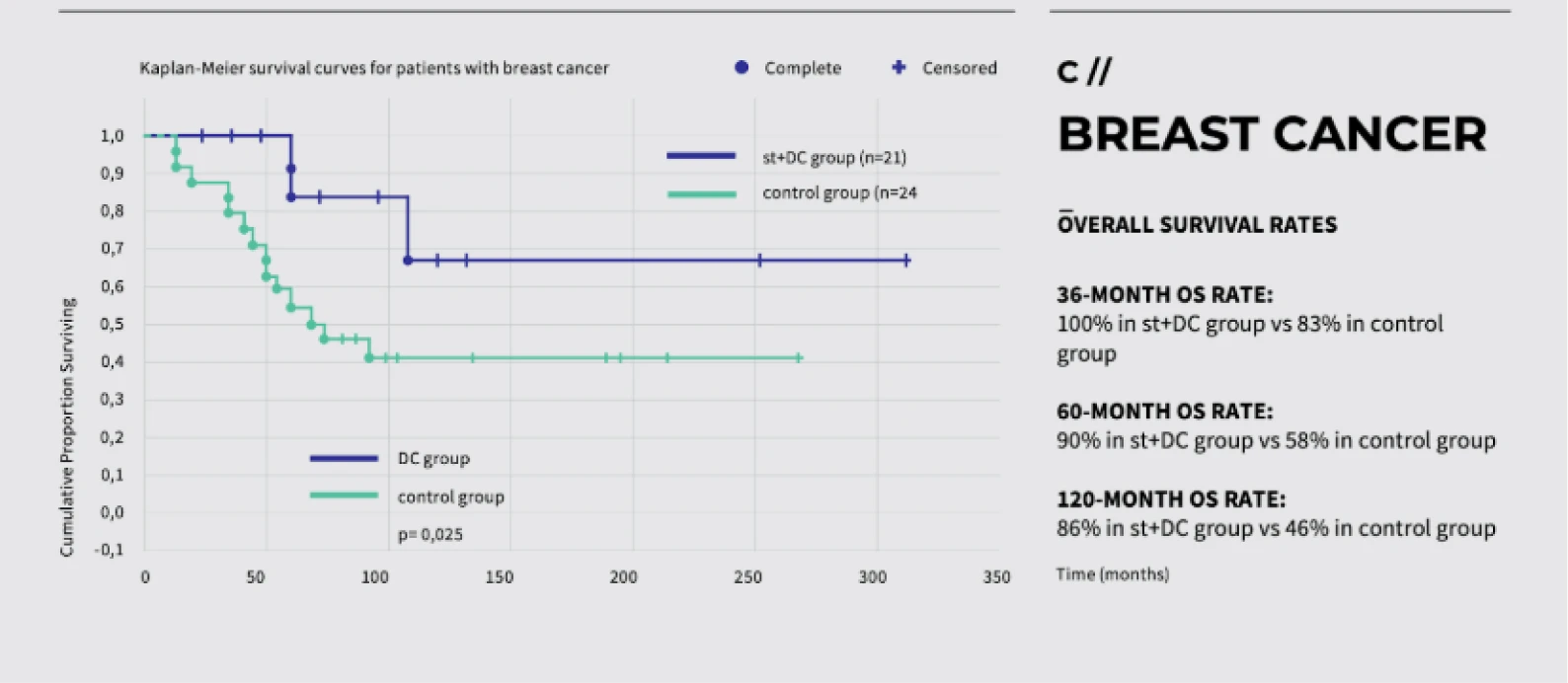
Dendritic cell therapy has shown promising results in clinical trials for melanoma, prostate cancer, and glioblastoma.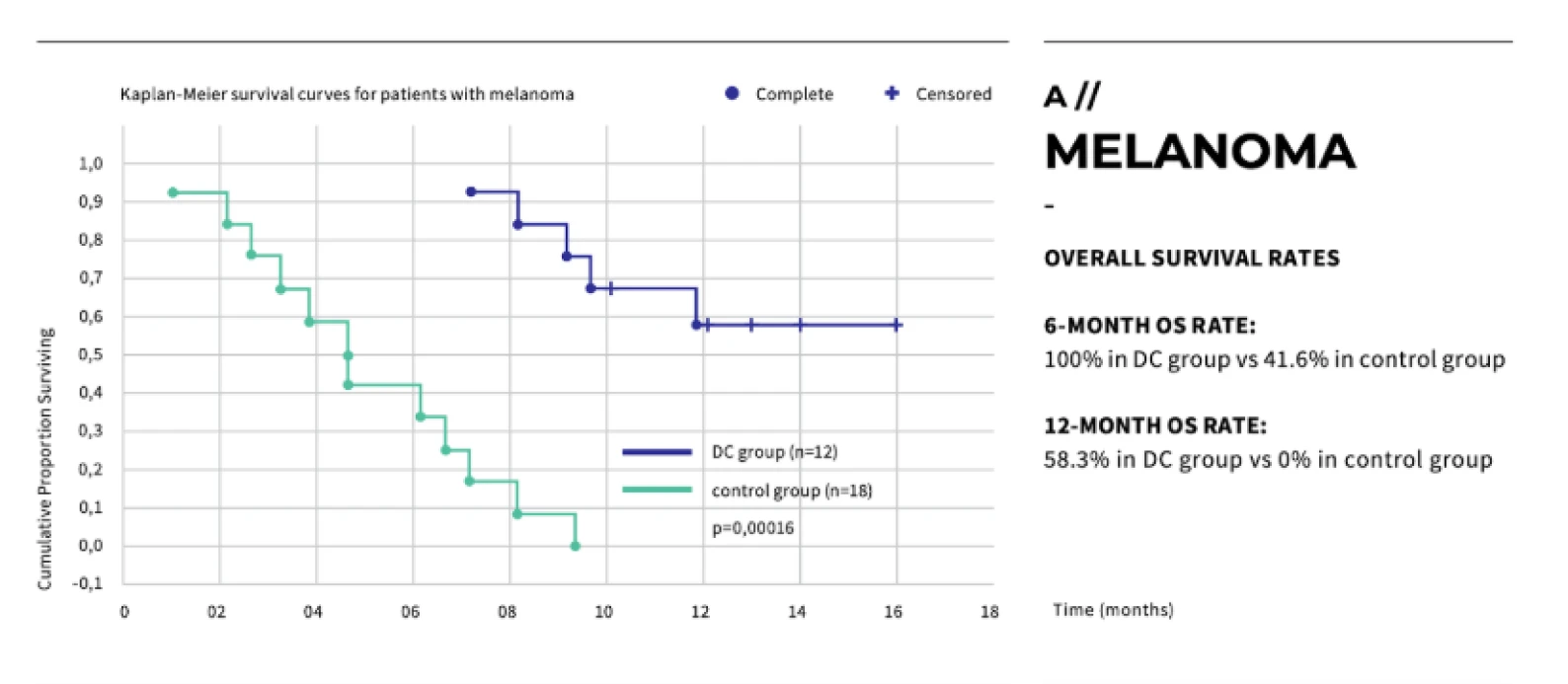
The Advantages and Disadvantages of Dendritic Cell Therapy
One of the key benefits of dendritic cell therapy is its personalised approach; the vaccine is developed using the patient’s own tissue, making it highly specific and significantly reducing the likelihood of side effects often associated with traditional treatments.
Additionally, dendritic cells can "train" other immune cells, providing long-lasting immune memory and protection even after the initial treatment phase. Another notable advantage is its cost-effectiveness. Sanctura’s dendritic cell therapy is more affordable than similar treatments in Europe, without compromising quality.
However, there are some challenges associated with dendritic cell therapy. A major limitation is the requirement for live tumour tissue, which must be cryogenically frozen or used directly to generate the vaccine. Furthermore, individuals with severely weakened immune systems may not respond as effectively to the therapy, limiting its suitability for some cases.
Oncolytic Viral Vaccines
Oncolytic Viral Vaccines
Oncolytic virus therapy is a form of immunotherapy that uses genetically modified viruses to target and destroy cancer cells while sparing healthy tissue. These viruses are engineered to infect cancer cells, where they replicate rapidly, causing the cells to burst open and die - a process known as oncolysis.
As the cancer cells are destroyed, they release tumour antigens, which stimulate the immune system to recognise and attack the remaining cancer cells. This dual action directly kills the tumour and trains the immune system to continue the fight.
How Does Oncolytic Virus Therapy Work?
Current research trends are expanding the use of oncolytic viruses in combination with other therapies, such as checkpoint inhibitors, to create powerful synergistic effects. Oncolytic virus therapy is showing promise in treating cancers such as melanoma, glioblastoma, breast cancer, and pancreatic cancer. Some of the most researched viruses include Talimogene Laherparepvec (T-VEC), which is FDA-approved for melanoma, and other engineered viruses targeting various tumour types.
The mechanism of action is both scientifically fascinating and emotionally inspiring. Once injected into the tumour, these engineered viruses ignite a chain reaction of destruction as if awakening the immune system from slumber.
The immune cells learn to see the cancer for what it is, relentlessly attacking it until the tumour shrinks and dies. This unique approach is revolutionising how we understand cancer treatment, turning viruses - once our enemies - into powerful allies in the fight against cancer.
The Advantages of Oncolytic Virus Therapy
Targeted Action
Oncolytic viruses specifically infect cancer cells, reducing damage to healthy tissue.
Immune System Boost
As cancer cells die, they release signals that help train the immune system to identify and destroy remaining tumour cells.
Combination Therapy
When used alongside other immunotherapies like checkpoint inhibitors, oncolytic viruses enhance the overall immune response and increase effectiveness.
The Disadvantages of Oncolytic Virus Therapy
Limited to Certain Cancers
The therapy is most effective in tumours that are accessible and have a permissive environment for viral infection.
Immune Clearance
Sometimes, the body’s immune system may clear the virus before it fully attacks the tumour.
Side Effects
Though generally well-tolerated, side effects can include flu-like symptoms and inflammation at the tumour site.
Top Viruses Used in Oncolytic Virus Therapy
Talimogene Laherparepvec (T-VEC)
Engineered herpes simplex virus, FDA-approved for treating melanoma.
Reovirus (Reolysin)
A naturally occurring virus being studied in various cancers, including head and neck, and ovarian cancer.
Adenovirus (ONYX-015)
A modified adenovirus that targets cancers like head and neck, colorectal, and pancreatic cancer.
Coxsackievirus (CVA21)
An oncolytic virus showing promise in clinical trials for melanoma and lung cancer.
Vaccinia virus (JX-594/Pexa-Vec)
Being tested in cancers such as liver cancer, with potential for broader use in combination therapies.
Peptide Vaccines
Peptide Vaccines
Peptide vaccines represent a cutting-edge approach in personalised immunotherapy, specifically designed to harness the body’s immune system to target cancer cells by identifying and attacking specific proteins found on the surface of your tumour.
At Sanctura, we work with CeGaT’s advanced peptide vaccine technology (FDA-approved) to create a customised treatment plan based on the unique molecular biology of each patient's tumour.
What is a Peptide Vaccine?
A peptide vaccine stimulates the immune system by introducing small protein fragments, known as neoantigens, derived from tumour cells. These neoantigens arise from DNA mutations specific to cancer cells, making them unique targets for therapy. By stimulating both cytotoxic T cells (CD8+ T cells) and helper T cells (CD4+ T cells), peptide vaccines help the immune system to recognise and destroy cancer cells more effectively.
The Treatment Process
The creation of a peptide vaccine begins with the whole exome sequencing of both tumour and normal tissue to identify somatic mutations specific to the cancer cells. These mutations, known as neoantigens, are then prioritised through hierarchical immunogenicity profiling to select the most promising targets for the vaccine.
How Peptide Vaccines Differ from Dendritic Cell Therapy
While dendritic cell vaccines involve collecting immune cells, modifying them in the lab, and reintroducing them into the body, peptide vaccines focus on delivering synthetic tumour-specific proteins (neoantigens) that stimulate the immune system to launch a targeted attack. This method relies less on manipulating immune cells and more on training the body to independently recognise and respond to abnormal peptides.
Clinical Research and Cancer Applications
Peptide vaccines have shown promising results in clinical trials, particularly in cancers such as melanoma, prostate cancer, and glioblastoma.
At Sanctura, we currently use CeGaT’s peptide vaccine technology to treat a wide range of cancers. Studies have demonstrated significant tumour shrinkage and longer progression-free survival in patients treated with peptide vaccines.
Why Choose CeGaT’s Peptide Vaccine at Sanctura?
We are proud to partner with CeGaT to offer their CancerNeo® peptide vaccine service. This highly specialised immunotherapy identifies tumour-specific mutations and creates a vaccine tailored to each patient’s unique cancer profile.
We work closely with CeGaT to comprehensively profile your tumour, determine vaccine candidacy, and provide collaborative monitoring of your condition and antibody levels through regular MDT meetings, ensuring the best possible outcomes in your fight against cancer.
Here’s what makes CeGaT’s CancerNeo® service special:
Whole Exome Sequencing
CeGaT's ExomeXtra® technology sequences both tumour and normal tissue, providing the highest resolution data for identifying somatic variants and mutations. This comprehensive analysis ensures that the vaccine targets only tumour-specific neoantigens.
HLA Typing and Neoantigen Prediction
HLA class I and II typing is performed to ensure that the selected neoantigens will effectively stimulate both CD8+ cytotoxic T cells and CD4+ helper T cells, crucial for a robust immune response.
Neoantigens are prioritised based on their immunogenic potential, with up to 12 neoepitopes chosen for the vaccine.
RNA Sequencing for Validation
Tumour whole RNA sequencing is used to confirm the expression of neoantigens at the RNA level, ensuring that the vaccine targets are present and active in the tumour cells.
Additional Services by CeGaT
Whole exome sequencing of tumour and normal tissue using CeGaT’s ExomeXtra®.
Assessment of somatic variants in over 700 tumour-relevant genes and fusions in over 30 genes.
Medical report with a validated list of variants, potential therapeutic relevance, and treatment options.
TMB determination, MSI prediction, and HRD score calculation.
Detecon of copy number variants (CNVs).
A list of all eligible drugs with EMA or FDA approval that match detected biomarkers in the tumour.
Pharmacogenetic analysis of germline variants affecting drug metabolism.
CHIP (Clonal Hematopoiesis of Indeterminate Potential) assessment.
Tumour whole RNA sequencing with rRNA depletion.
HLA typing.
Prediction of HLA class I and class II-restricted peptide epitopes from sequencing data.
Second medical report with selected peptides for vaccine formulation.
RNA-based fusion transcript analysis (CancerFusionRx®) covering over 150 genes for fusion detection (optional service).
Immunohistochemical (IHC) analyses, including PD-L1 staining and CAR-T cell panel analysis (optional service).
MGMT promoter methylation analysis for glioma patients to assess chemotherapy sensitivity (optional service).
The Advantages of Peptide Vaccines
Peptide vaccines are highly personalised to your specific cancer. Collaborative monitoring through multidisciplinary team (MDT) meetings between Sanctura and the physician team at CeGaT also ensures that your treatment is continually optimised.
Additionally, the vaccine induces a long-lasting immune response, with antibodies that can remain effective for several years. Leveraging cutting-edge immunotherapy, CeGaT’s technology ensures the vaccine stimulates cytotoxic and helper T cells, improving the immune system's ability to combat cancer effectively. Moreover, peptide vaccines are safe and tumour-specific, with minimal side effects compared to other treatments.
The Disadvantages of Peptide Vaccines
A key limitation is the need for high-quality tumour tissue to create the vaccine, which can be challenging to obtain. The vaccine's effectiveness also depends on the patient’s immune system, which may be compromised in advanced cancer stages.
Furthermore, the treatment currently requires travel to Germany for vaccine production and administration, though efforts are underway to establish vaccine centres in Cape Town and London (pending regulatory approvals). Another consideration is the significant financial cost of peptide vaccines.
Latzer, P., Zelba, H., Battke, F., et al. (2024). A real-world observation of patients with glioblastoma treated with a personalised peptide vaccine. Nature Communications, 15, 6870. https://www.nature.com/articles/s41467-024-51315-8
CAR-T Therapy
CAR-T Therapy
CAR-T therapy, or Chimeric Antigen Receptor T-cell therapy, is a form of immunotherapy that uses a patient's immune cells to fight cancer precisely.
How Does CAR-T Therapy Work?
T-cells are extracted from the blood and genetically modified to express special receptors (CARs) that recognise and target specific proteins in cancer cells.
These engineered T-cells are then reinfused into the body, where they seek out and bind to cancer cells, triggering a powerful immune response that destroys the tumour. Once reintroduced, these T-cells continue to multiply and train other immune cells to boost the body's defence against cancer. The process begins with collecting T-cells from the patient’s blood.
These T-cells are then modified in a laboratory where specific gene sequences are inserted to produce chimeric antigen receptors (CARs). These CARs protrude on the surface of the cancer, killing T-cells. They have been engineered to recognise specific proteins in cancer cells and attach to them, ultimately unleashing a lethal cell response and killing the tumour cell. Once these T-cells are reintroduced into the patient’s body, they can continue to multiply and train other cancer-fighting T-cells, which are now better equipped to find, bind to, and eliminate cancer cells.
What Does CAR-T Therapy Do?
CAR-T therapy has been particularly effective in treating certain forms of leukaemia and lymphoma, where remarkable remission rates have been achieved. Clinical trials are expanding its use to solid tumours, including cancers like glioblastoma and pancreatic cancer, where ongoing research aims to refine its efficacy in these more challenging cases.
CAR-T therapy is currently approved in many countries, including the United States, United Kingdom, China, and several European nations, with stringent requirements for patient eligibility, including detailed assessments of the cancer type and stage. Some therapies, like Kymriah and Yescarta, are available for certain lymphomas and leukaemias.
The Advantages of CAR-T Therapy
High Precision
CAR-T therapy is highly specific to cancer cells, reducing damage to healthy tissue.
Durable Response
CAR-T cells remain active in the body, offering prolonged protection and potential long-term remission.
Rapid Effectiveness
Tumours have sometimes shrunk within days following treatment.
The Disadvantages of CAR-T Therapy
Cost
CAR-T therapy is extremely expensive and not widely available in all countries.
Potential for Severe Side Effects
These include cytokine release syndrome (CRS) and neurotoxicity, which can be serious but are often manageable with medical intervention.
Eligibility Requirements
CAR-T therapy is only suitable for certain cancer types, and patients must meet specific medical criteria to undergo treatment.
Is CAR-T Therapy Right For You?
At Sanctura, we offer consultations on whether CAR-T therapy may suit your cancer and a screening process of your tumour subtype for possible experimental CAR-T therapy. Many patients have experienced profound remission with this revolutionary treatment, even in advanced and difficult-to-treat cases.
Speak with your oncologist to explore if CAR-T therapy could be part of your cancer journey.
